Research Areas
Stem Cell Differentiation
We use stem cells to understand basic cell biology, disease mechanisms, and potential treatments. We culture stem cells in the lab and guide their differentiation to produce specialized cells such as nerve, heart, or skin cells. Through controlled experiments, we study how stem cells repair tissues, respond to injury, and interact with biomaterials.
We also develop stem cell–based therapies, test their safety and effectiveness in disease models, and design clinical trials for patient treatment. Our work combines cell biology, regenerative medicine, tissue engineering, and advanced lab techniques to translate stem cell science into real-world medical solutions.
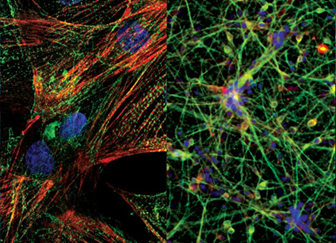
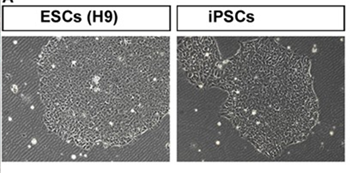
Induced pluripotent stem cells (iPSCs) are adult cells that have been genetically reprogrammed to revert to a pluripotent state, meaning they can develop into almost any cell type in the body. iPSCs are created by introducing specific genes into somatic cells, allowing researchers to generate patient-specific stem cells for disease modeling, drug testing, and regenerative therapies.

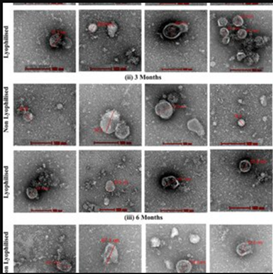
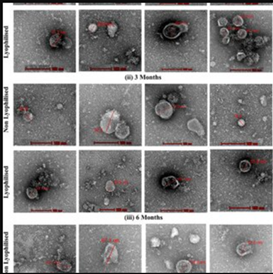
Extracellular Vesicles
Extracellular vesicles (EVs) are tiny, membrane-bound particles released by cells into their surrounding environment. They carry proteins, lipids, RNA, and other molecules, and act as messengers for cell-to-cell communication. There are different types of EVs, mainly exosomes and microvesicles, which vary in size and origin.
In research, EVs are studied for their roles in physiological processes, such as tissue repair and immune modulation, as well as their involvement in diseases. Scientists isolate and characterize EVs to explore their functions and therapeutic potential, including using EVs from stem cells to promote regeneration and deliver targeted treatments. Their ability to transfer signals between cells makes EVs a promising tool in diagnostics and regenerative medicine.
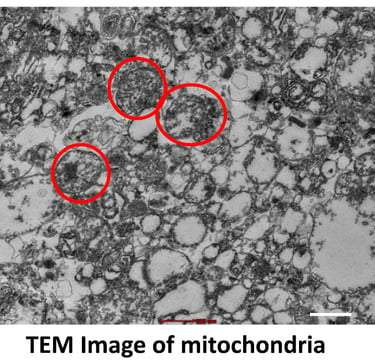
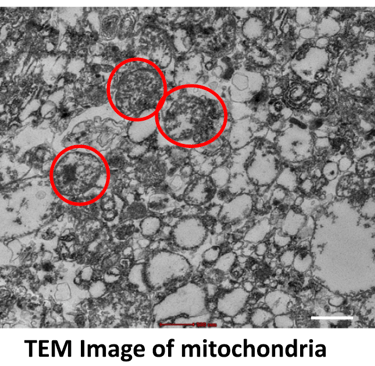
Mitochondria play a vital role in regenerative medicine because they are the cell’s powerhouse, producing energy necessary for growth, repair, and survival. Healthy mitochondrial function is crucial for stem cell viability, differentiation, and tissue regeneration.
In research, scientists study how mitochondria support stem cells and repair processes, investigate mitochondrial dysfunction in diseases, and explore therapies such as mitochondrial transplantation, enhancement, or protection to improve tissue healing. Modulating mitochondrial activity can increase the regenerative capacity of cells, influence cell fate, and improve the success of stem-cell–based treatments in conditions like neurodegeneration, cardiac injury, and metabolic diseases.
3D bioprinting is an advanced technology that uses layer-by-layer printing to create living tissues and organ-like structures. It combines cells, biomaterials, and growth factors in precise patterns, mimicking the architecture of natural tissues.
In regenerative medicine research, 3D bioprinting allows us to produce complex tissue models—such as skin, cartilage, or even parts of organs—for studying disease, testing drugs, and developing future therapies. Scientists use bioprinters to fabricate scaffolds that support cell growth and differentiation, offering personalized solutions for tissue repair and transplantation. The technology promises improved tissue integration, faster healing, and breakthroughs in organ replacement and drug screening.


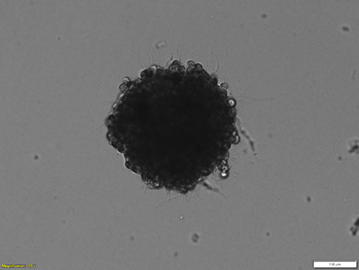
Organoid culture is a cutting-edge technique where stem cells are grown in 3D, allowing them to self-organize into miniaturized, simplified versions of organs called organoids. These organoids mimic essential features of real tissues—such as the brain, liver, intestine, or kidney—providing a powerful platform for research.
Scientists use organoid culture to study organ development, model diseases, test drugs, and explore personalized medicine. Organoids allow us to investigate human biology more accurately than traditional cell cultures and have become invaluable tools for understanding genetic disorders, infectious diseases, and regenerative therapies.